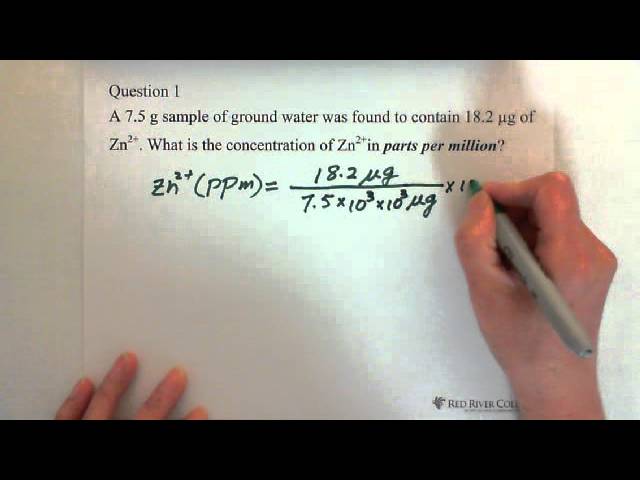The influence of Pt dopant concentration in WO3 on the: (A) measured... | Download Scientific Diagram

SOLVED: A solution containing 4.03 ppm XYar had a transmittance of 0.123 in a 1.00-cm cell at 460 nU Calculate the molar absorptivity of XYa, at 460 nm What is the order

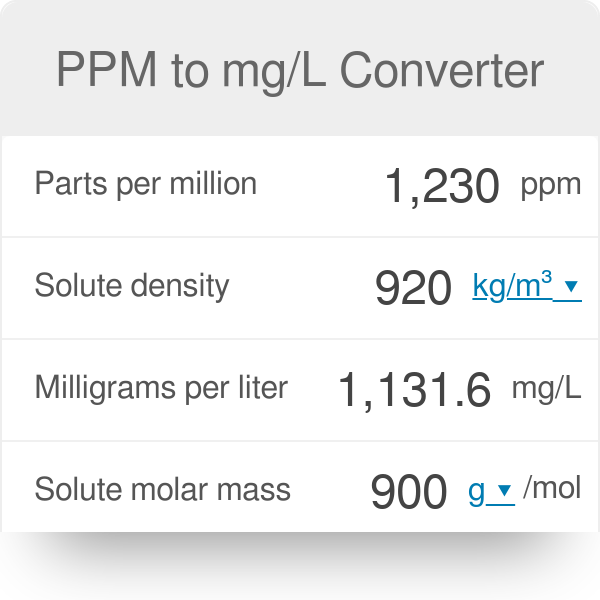
A sample of drinking water was found to be severely contaminated with chloroform (CHCl3) supposed to be a carcinogen. The level of contamination was 15 ppm (by mass):(i) express this in percent

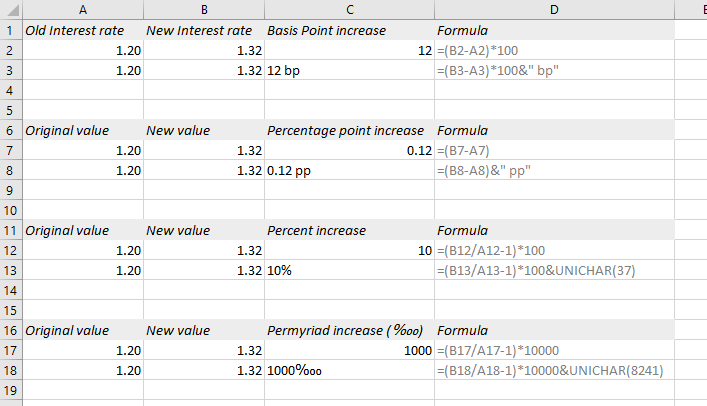
SOLVED: Percent by definition is parts per hundred 4 % (wlw) (mass of solutelmass of solution) x 100 For very dilute solutions, chemists will often use parts per thousand or parts per
Amounts of beer, wine. liquor and total consumption calculated into... | Download Scientific Diagram

Solved) - Percent by definition is parts per hundred. % (w/w) = (mass of... (1 Answer) | Transtutors

SOLVED: Percent by definition is parts per hundred: % (wlw) (mass of solutelmass of solution) x 100. For very dilute solutions, chemists will often use parts per thousand or parts per million (
GitHub - zesteros/ArduinoAirQualityMonitor: App for gas monitoring with MQ135 Gas sensor, Android and Arduino