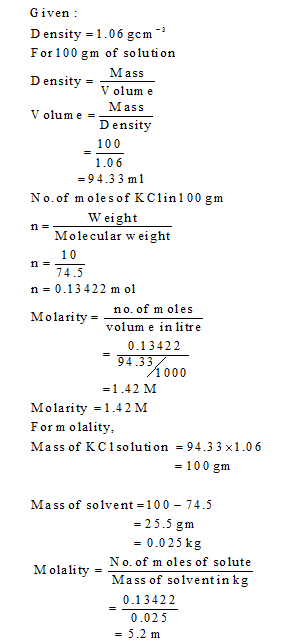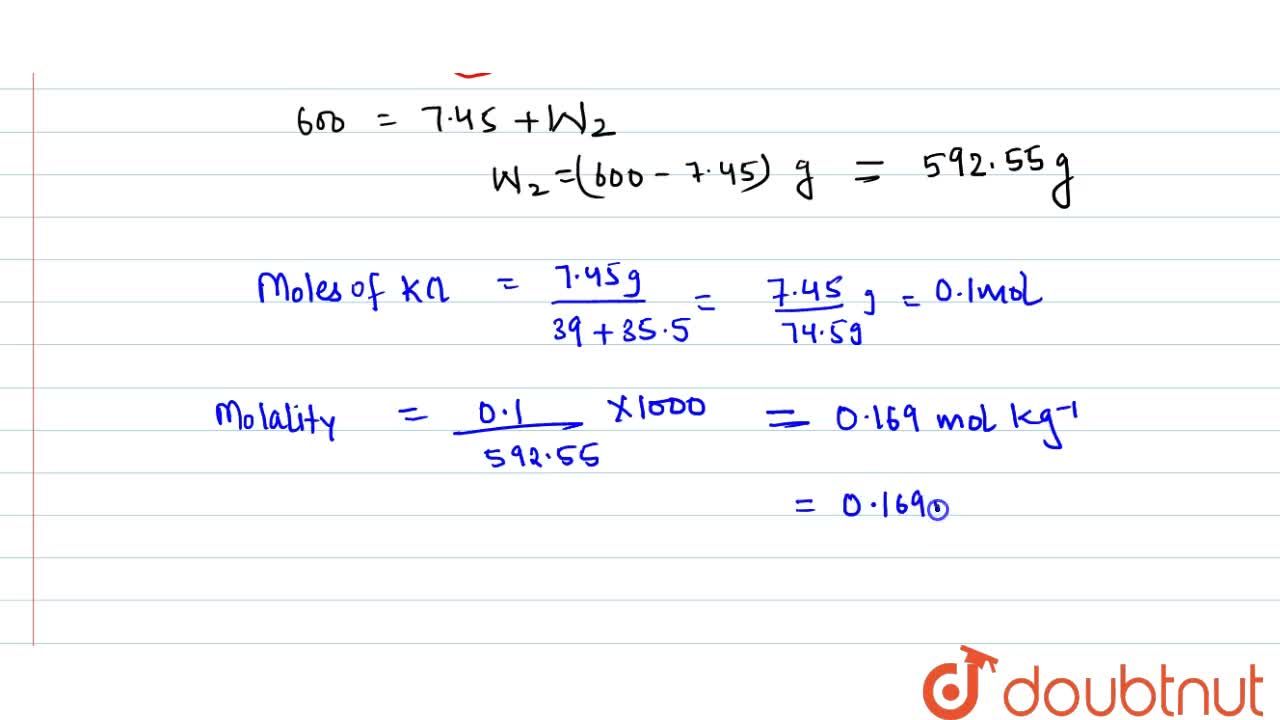
Calculate the molarity of KCl solution prepared by dissolving 7.45 g of KCl in 500 mL of the solution. (d(sol) = 1.2 g mL^(-1))
Processes | Free Full-Text | Effect of Partial Substitution of Sodium Chloride (NaCl) with Potassium Chloride (KCl) Coupled with High-Pressure Processing (HPP) on Physicochemical Properties and Volatile Compounds of Beef Sausage under

Quantification of Zeta-Potential and Electrokinetic Surface Charge Density for Colloidal Silica Nanoparticles Dependent on Type and Concentration of the Counterion: Probing the Outer Helmholtz Plane | The Journal of Physical Chemistry C

The density of `3M` solution of `NaCl` is `1.25 g mL^(-1)`. The molality of the solution is... - YouTube
SOLVED: What is the Molar concentration of a solution with a volume 3.3 mL that contains 12 grams of ammonium sulfite? How many grams of copper (II) fluoride are needed to make

Calculate the molality of the KOH solution having density 1.5 g/mL, when the molarity of the same - Brainly.in

A solution of KCl has a density of 1.69 g mL^(-1) and is 67% by weight. Find the denisty of the solution if it is diluted so that the percentage by weight

Utilization of Low-Concentration CO2 with Molecular Catalysts Assisted by CO2-Capturing Ability of Catalysts, Additives, or Reaction Media | Journal of the American Chemical Society

Ketamine decreases neuronally released glutamate via retrograde stimulation of presynaptic adenosine A1 receptors | Molecular Psychiatry

The density of a 10.0% by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.

Measurements of Vapor Pressures of Aqueous Solutions in the NaCl–KCl–H2O System from 493.15 to 693.25 K in a Fused Silica Capillary High-Pressure Optical Cell | Journal of Chemical & Engineering Data
Change in the concentration of silicon-containing ions in the KCl melt... | Download Scientific Diagram

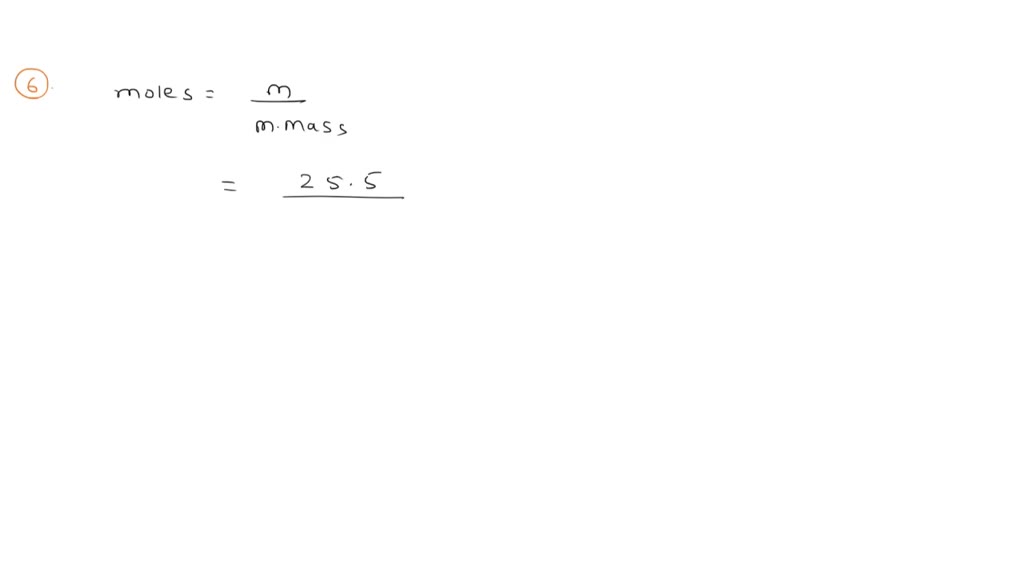
A solution is prepared by dissolving 4 g of NaOH to give 500 ml of it. Calculate the molality of the solution.

Calculate the molarity and molality of 20% aqueous ethanol (C2H5OH) solution by volume. (Density of solution = 0.96 g/mL)

A solution of `KCl` has a density of `1.69 g mL^(-1)` and is 67% by weight. Find the denisty of ... - YouTube