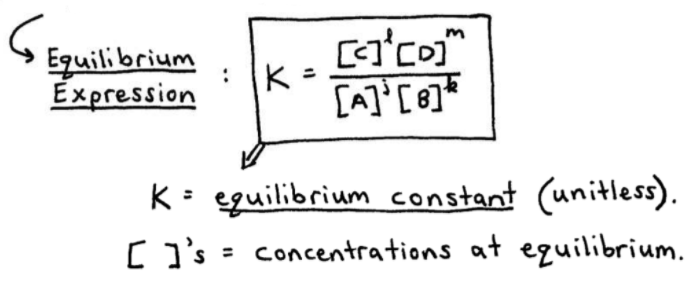
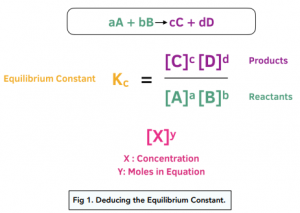
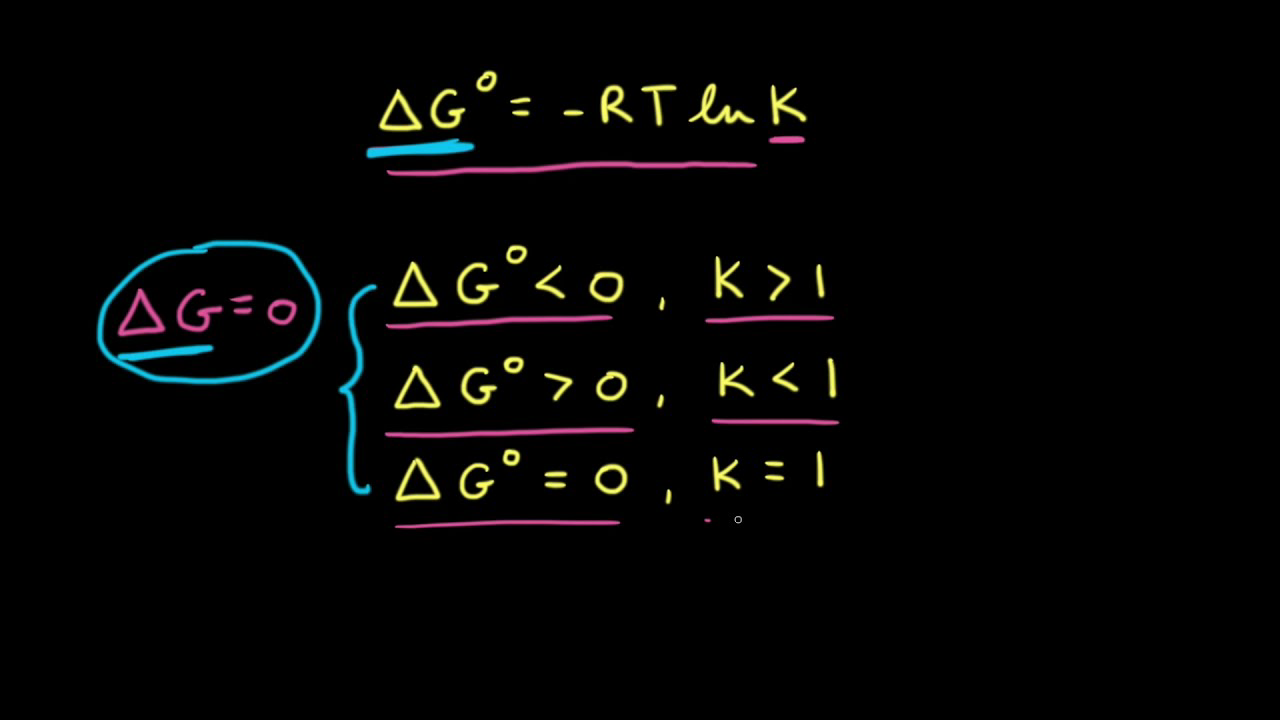OneClass: Calculate Kp for the reaction below. ( Need both A and B) MasteringChemistry: MCHW Ch14A Se...

Calculate the equation constant for the reaction: H2 (g) + CO2 (g) H2O (g) + CO at 1395 K, if the equilibrium constants at 1395 K for the following are 2H2O (g)
![Calculate Kp for the reaction, C(s) + H2O(g) CO(g) + H2(g) at 990 K if the equilibrium concentration are as follows : [H2O] = 1.10 M, [CO] = [H2] = 0.2 M, Calculate Kp for the reaction, C(s) + H2O(g) CO(g) + H2(g) at 990 K if the equilibrium concentration are as follows : [H2O] = 1.10 M, [CO] = [H2] = 0.2 M,](https://dwes9vv9u0550.cloudfront.net/images/6653152/88b9752c-8929-4745-bc8f-8705cb2ad9b9.jpg)
Calculate Kp for the reaction, C(s) + H2O(g) CO(g) + H2(g) at 990 K if the equilibrium concentration are as follows : [H2O] = 1.10 M, [CO] = [H2] = 0.2 M,

Calculating equilibrium constants from equilibrium concentrations or partial pressures (worked examples) (video) | Khan Academy

Question Video: Calculating the Equilibrium Constant for Concentration Given the Initial Amount of Each Reactant | Nagwa

Lecture 7 Chemical Equilibrium Define equilibrium constant – K Define the free energies: G f , G r and G r Calculate K from G r Define Q – - ppt download