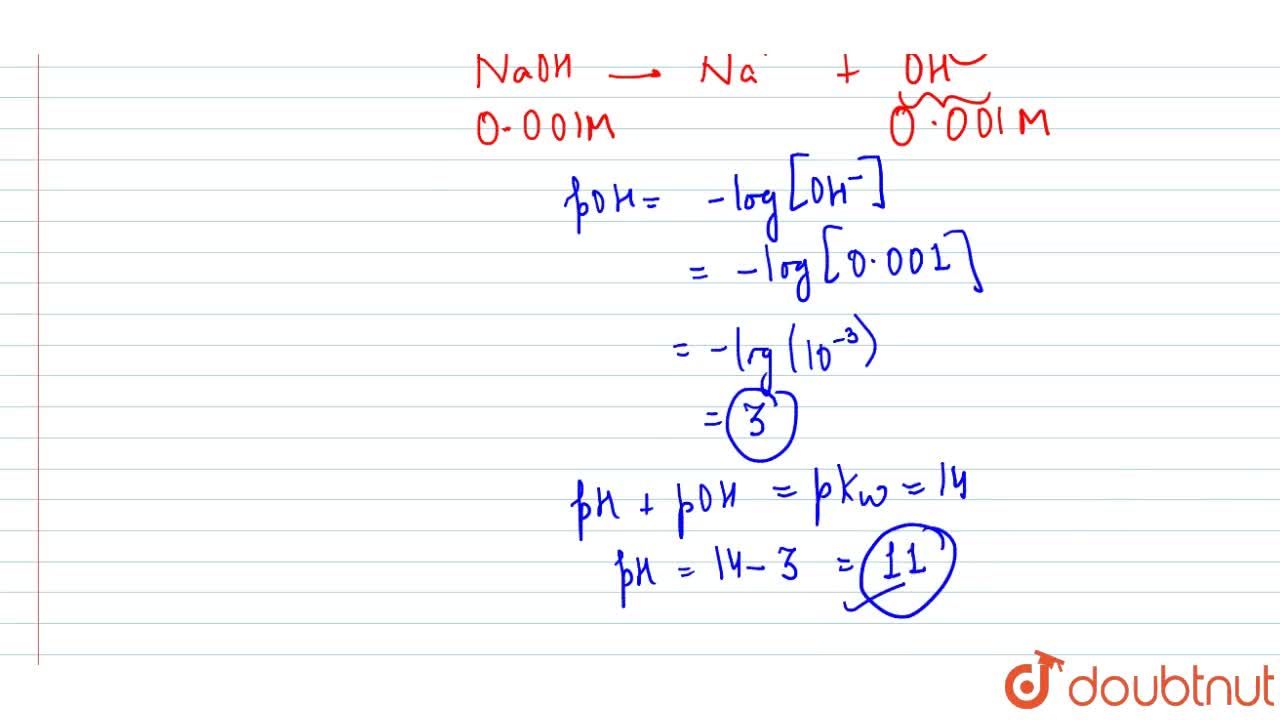Calculate pH for: (a) `0.001 NaOH`, (b) `0.01N Ca(OH)_(2)`, (c ) `0.01M Ca(OH)_(2)`, (d) `10^(-8 - YouTube
Calculate pH for : (a) 0.001 N NaOH, (b) 0.01 N Ca(OH)2 - Sarthaks eConnect | Largest Online Education Community
Calculate pH for : (a) 0.001 N NaOH, (b) 0.01 N Ca(OH)2 - Sarthaks eConnect | Largest Online Education Community
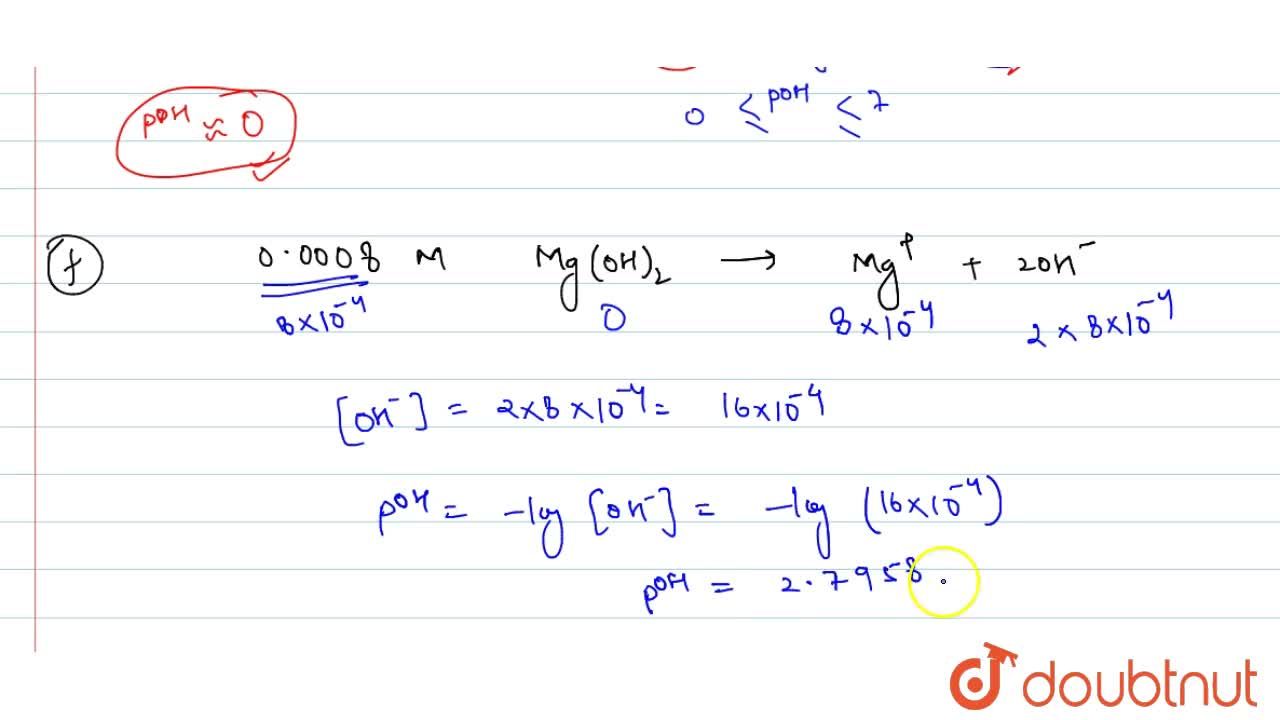
Calculate pH for: (a) 0.001 NaOH, (b) 0.01N Ca(OH)(2), (c ) 0.01M Ca(OH)(2), (d) 10^(-8)M NaOH, (e ) 10^(2)MNaOH, (f) 0.0008MMg(OH)(2) Assume complete ionisation of each.

Assuming complete dissociation, calculate the pH of the following solutions:(a) 0.003 M HCl (b) 0.005 M NaOH (c) 0.002 M HBr (d) 0.002 M KOH

Topic 18- Acids and bases 18.1 Calculations involving acids and bases 18.2 Buffer solutions 18.3 Salt hydrolysis 18.4 Acid-base titrations 18.5 Indicators. - ppt video online download






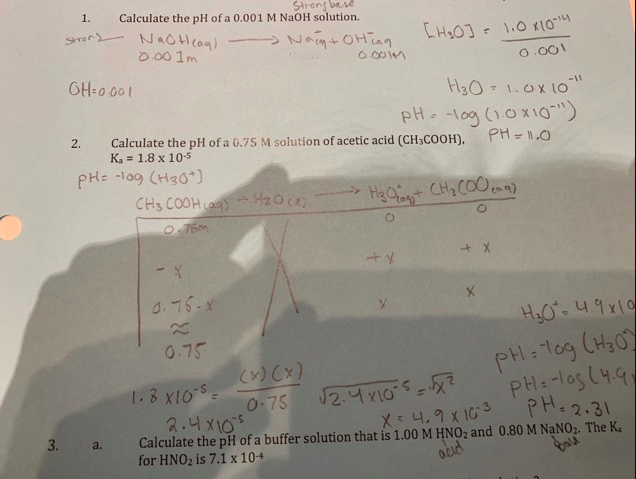
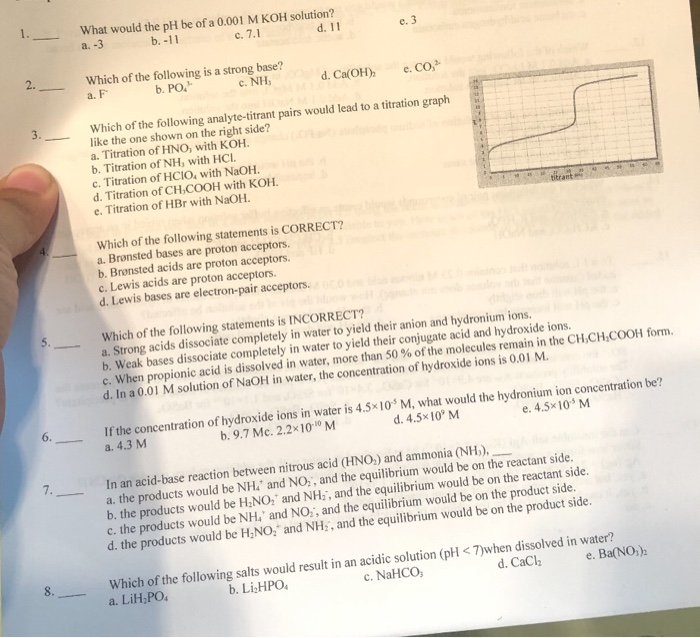






![Solved Calculation of pH, pOH, [H^+] of Acids and Bases | Chegg.com Solved Calculation of pH, pOH, [H^+] of Acids and Bases | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2Fc1f%2Fc1f7b306-69df-4e25-b874-3a1184640a55%2Fimage)