Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

In Situ Raman Spectroscopic Study of Gypsum (CaSO4·2H2O) and Epsomite (MgSO4·7H2O) Dehydration Utilizing an Ultrasonic Levitator | The Journal of Physical Chemistry Letters

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega

Reaction Characteristics of CaSO4/CaSO4·1/2H2O Reversible Reaction for Chemical Heat Pump | Semantic Scholar

Structures of a) gypsum, b) hemihydrate, and c) insoluble anhydrite... | Download Scientific Diagram

Experimental study of the dehydration reactions gypsum-bassanite and bassanite-anhydrite at high pressure: indication of anomalous behavior of H(2)O at high pressure in the temperature range of 50-300 degrees C. | Semantic Scholar
Investigations on dehydration and rehydration processes in the CaSO4 – H2O system at controlled time, temperature and humidity

Preparation and Application in HDPE of Nano-CaSO4 from Phosphogypsum | ACS Sustainable Chemistry & Engineering

Experimental Study of Hydration/Dehydration Behaviors of Metal Sulfates M2(SO4)3 (M = Sc, Yb, Y, Dy, Al, Ga, Fe, In) in Search of New Low-Temperature Thermochemical Heat Storage Materials | ACS Omega

SEM of calcium sulfate (gypsum) precipitate in presence of additive... | Download Scientific Diagram

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega
A crystallographic study of the low-temperature dehydration products of gypsum, CaSOa ' 2H2Oz hemihydrate CaSOr ' 0.50H2O, and 1

Reaction control of CaSO4 during hydration/dehydration repetition for chemical heat pump system - ScienceDirect
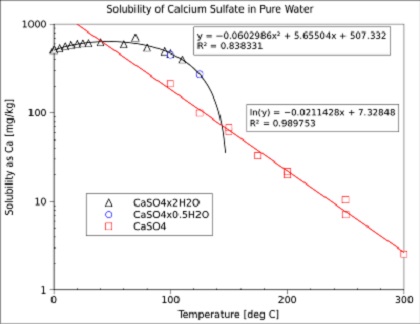
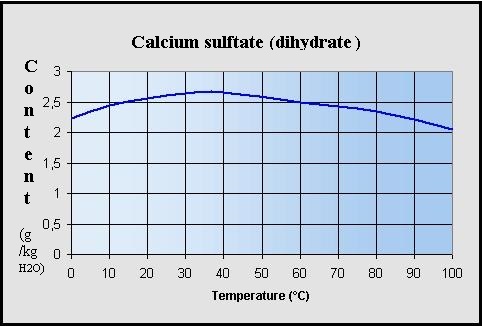
Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K

Figure 1 from In Situ Raman Spectroscopic Study of Gypsum (CaSO4·2H2O) and Epsomite (MgSO4·7H2O) Dehydration Utilizing an Ultrasonic Levitator. | Semantic Scholar

The Influence of Impurities on the Dehydration and Conversion Process of Calcium Sulfate Dihydrate to α-Calcium Sulfate Hemihydrate in the Two-Step Wet-Process Phosphoric Acid Production | ACS Sustainable Chemistry & Engineering

Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4 | ACS Omega
Comprehensive Thermodynamic Study of the Calcium Sulfate–Water Vapor System. Part 1: Experimental Measurements and Phase Equil