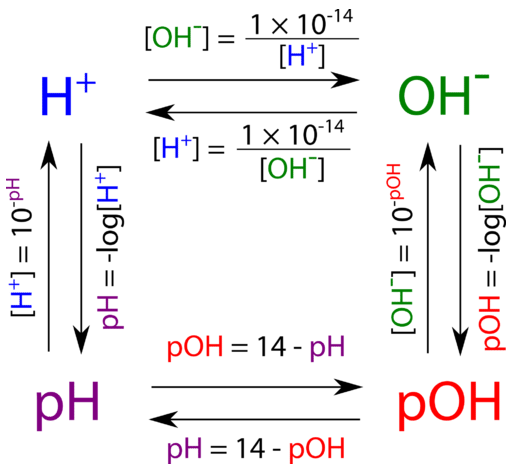
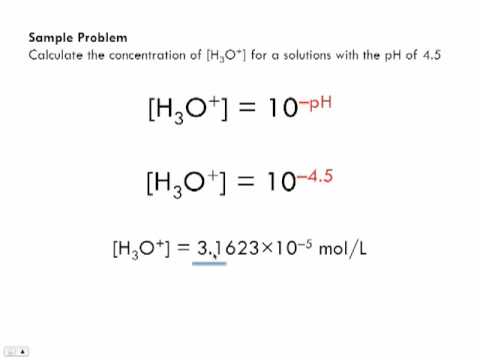
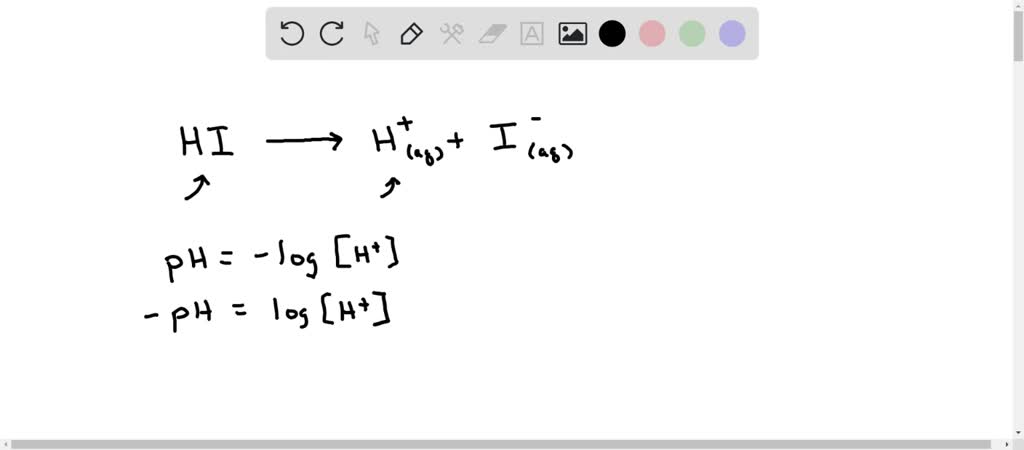
Calculate the hydrogen ion concentration in the following biological fluids whose pH are given below:(a) Human muscle - fluid, 6.83 (b) Human stomach fluid, 1.2 (c) Human blood, 7.38 (d) Human saliva, 6.4.
![Calculating pH and pOH. pH pH = - log [H + ] [H + ] = the hydrogen ion concentration pH: “potential of hydrogen” - A way of expressing the hydrogen ion. - ppt download Calculating pH and pOH. pH pH = - log [H + ] [H + ] = the hydrogen ion concentration pH: “potential of hydrogen” - A way of expressing the hydrogen ion. - ppt download](https://images.slideplayer.com/17/5302527/slides/slide_3.jpg)
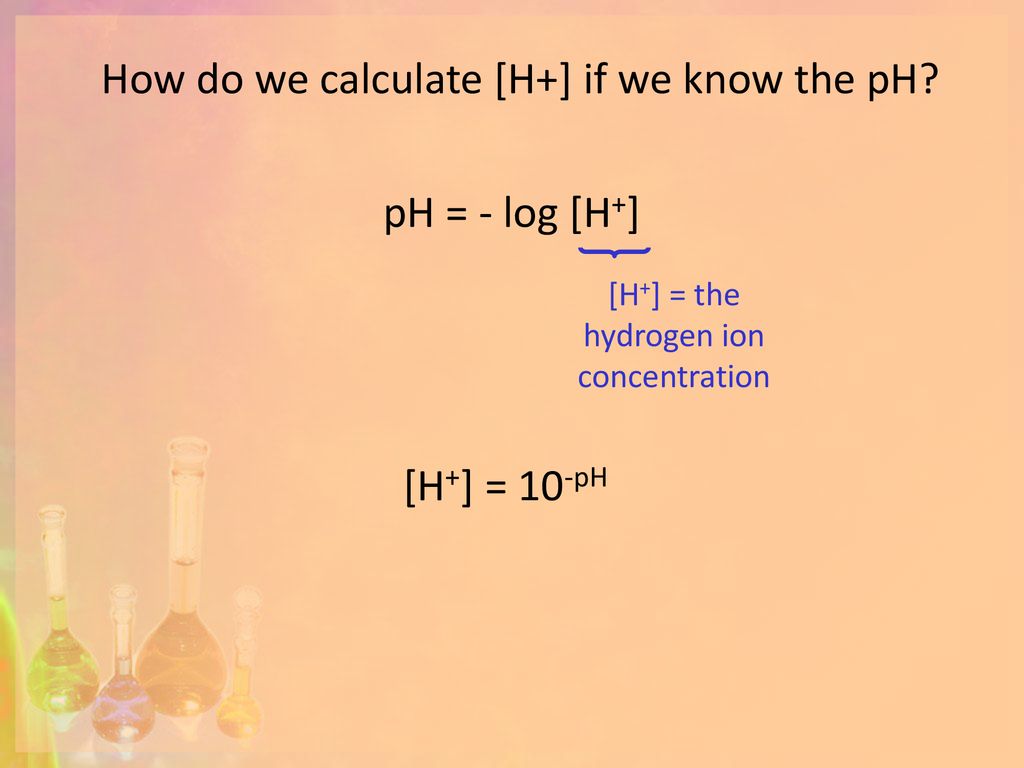
Calculating pH and pOH. pH pH = - log [H + ] [H + ] = the hydrogen ion concentration pH: “potential of hydrogen” - A way of expressing the hydrogen ion. - ppt download
The pH of an acetic acid solution is 3.26. What is the concentration of acetic acid and what is the percent of acid that's ionized? - Quora

![Calculating [OH-], pH and pOH from Kb Calculating [OH-], pH and pOH from Kb](https://www.mi.mun.ca/users/pfisher/chemistry1011_135/img007.gif)


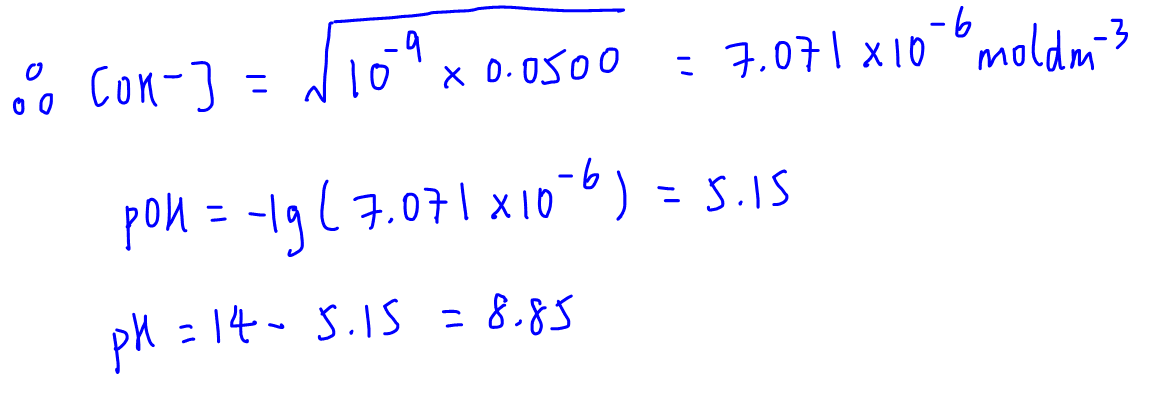
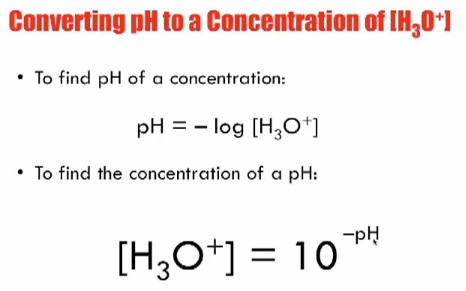
:max_bytes(150000):strip_icc()/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)


![Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube](https://i.ytimg.com/vi/gn1CgBzShps/maxresdefault.jpg)

![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)

![Calculations of pH, pOH, [H+] and [OH-] Calculations of pH, pOH, [H+] and [OH-]](https://www.sciencegeek.net/Chemistry/taters/graphics/pHSchematic.gif)