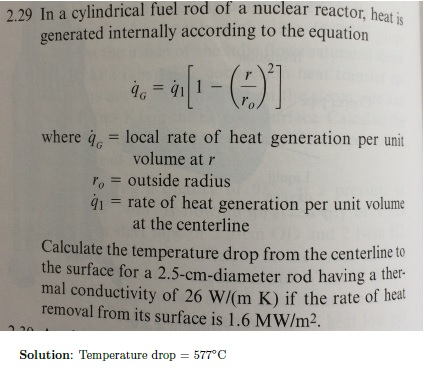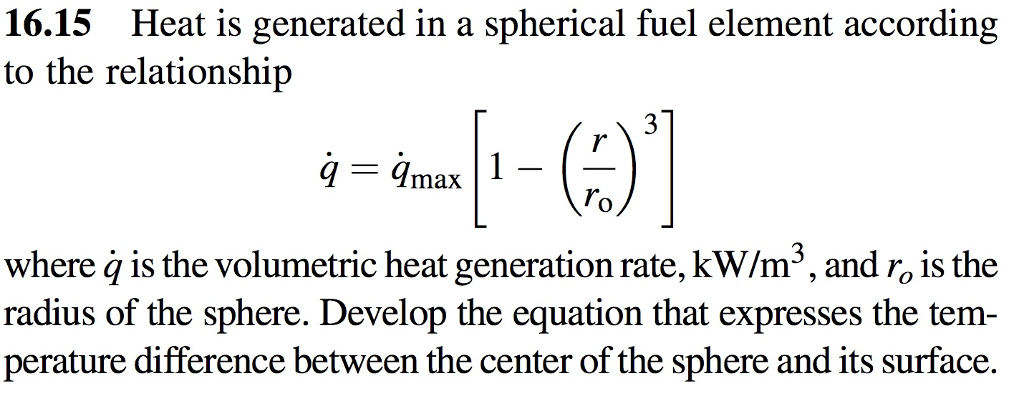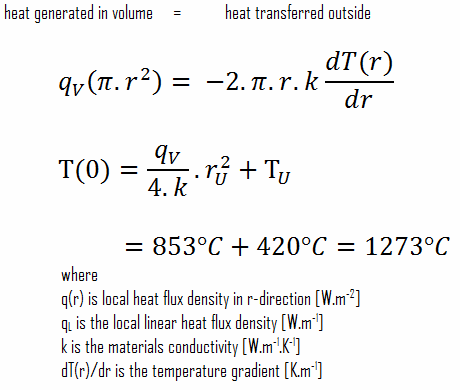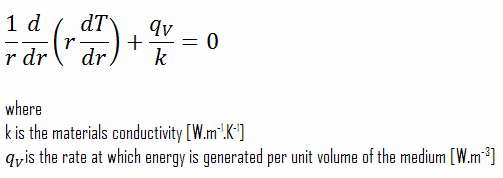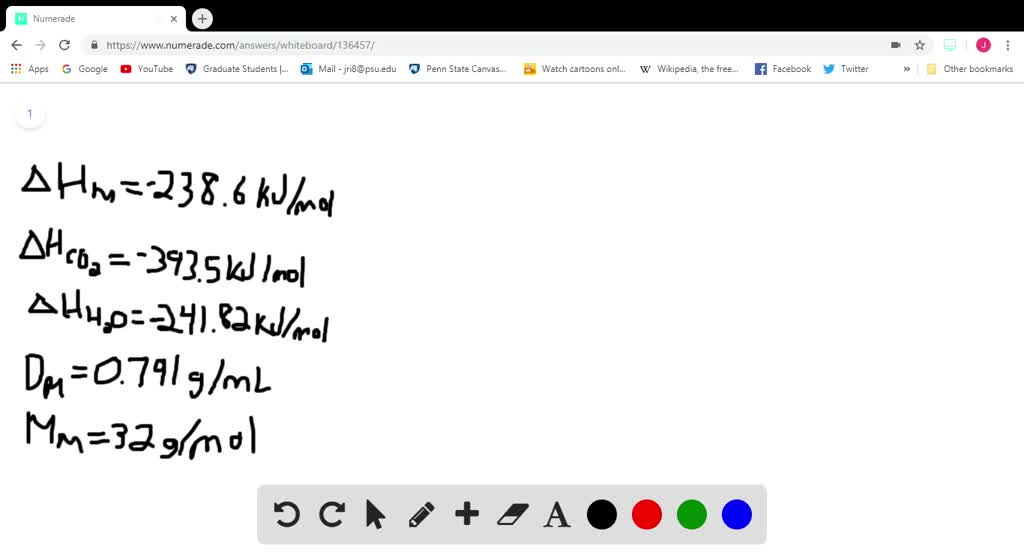
SOLVED:Methanol (CH3 OH) is used as a fuel in race cars. (a) Write a balanced equation for the combustion of liquid methanol in air. (b) Calculate the standard enthalpy change for the

Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy | Industrial & Engineering Chemistry Research

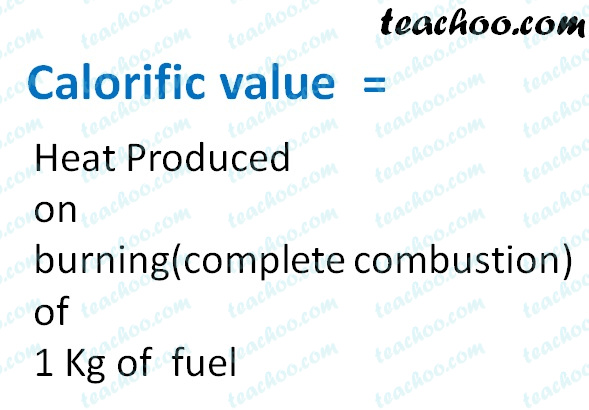
Q11 In an experiment 45 kg of a fuel was completely burnt The heat produced was measured to be 18000...

In an experiment, 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,00