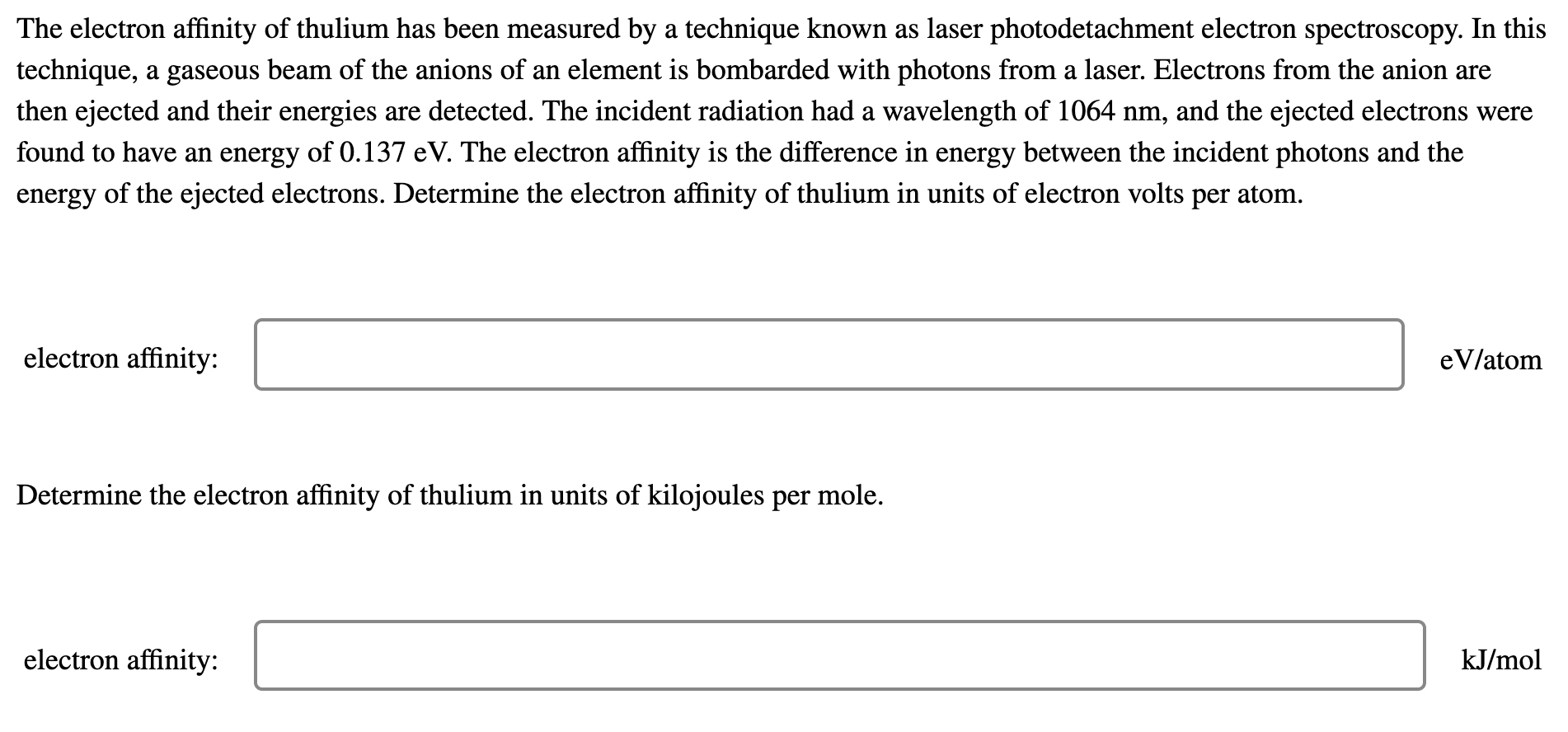
English) Calculate the energy of one mole of photons of radiations whose frequency is 5 X 10^ 14 Hz - YouTube
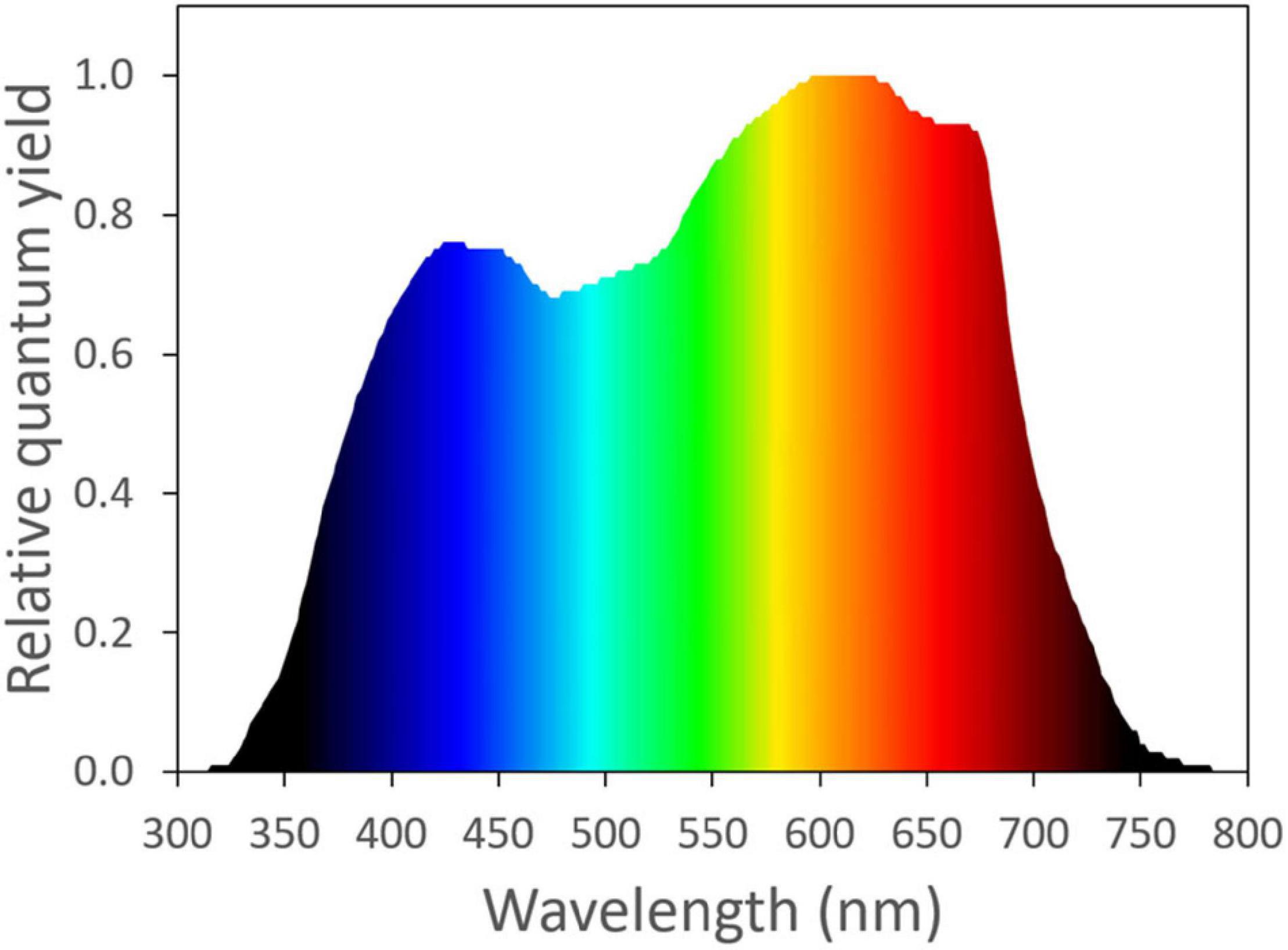
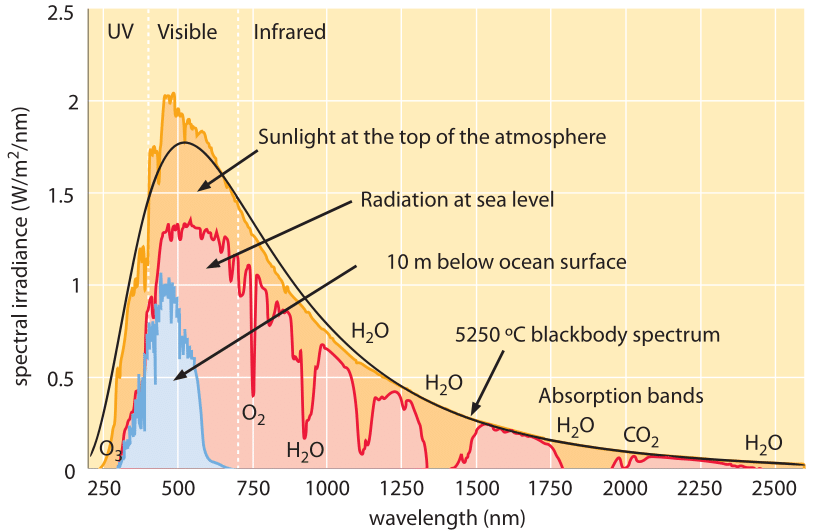
Frontiers | Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms
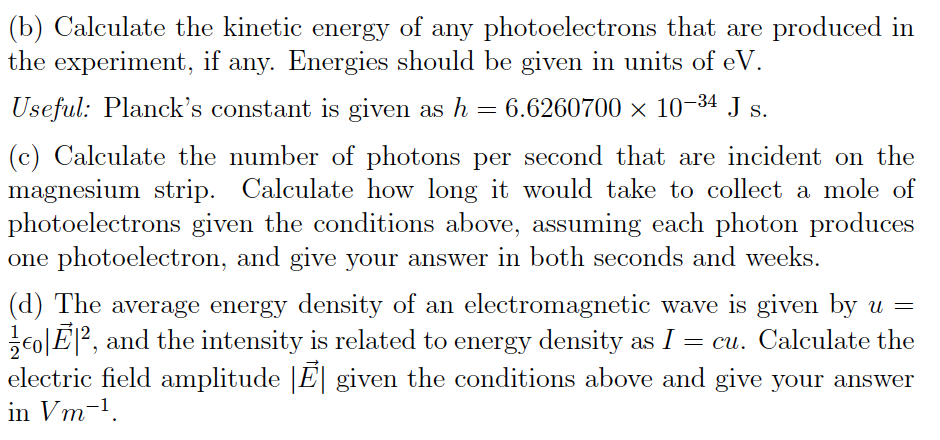
With what maximum kinetic energy will the electrons be ejected when the metal is exposed to light with a wavelength of 285 nm? - Quora

Calculate the energy in kilocalorie per mole of photons of an electromagnetic radiation having a wavelength of 7600 A.A. 33.56B. 37.56C. 47.35D. 42.35
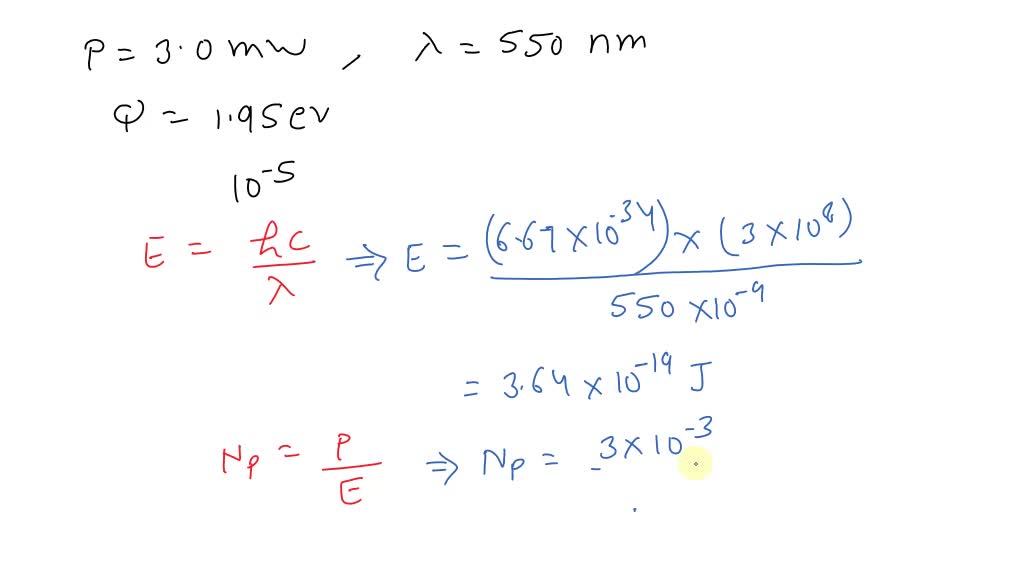
SOLVED: A radiation of 253 nm incident on HI results in the decomposition of 1·85×10 -2 mole per 1000 cals of radiant energy. Calculate the quantum efficiency.

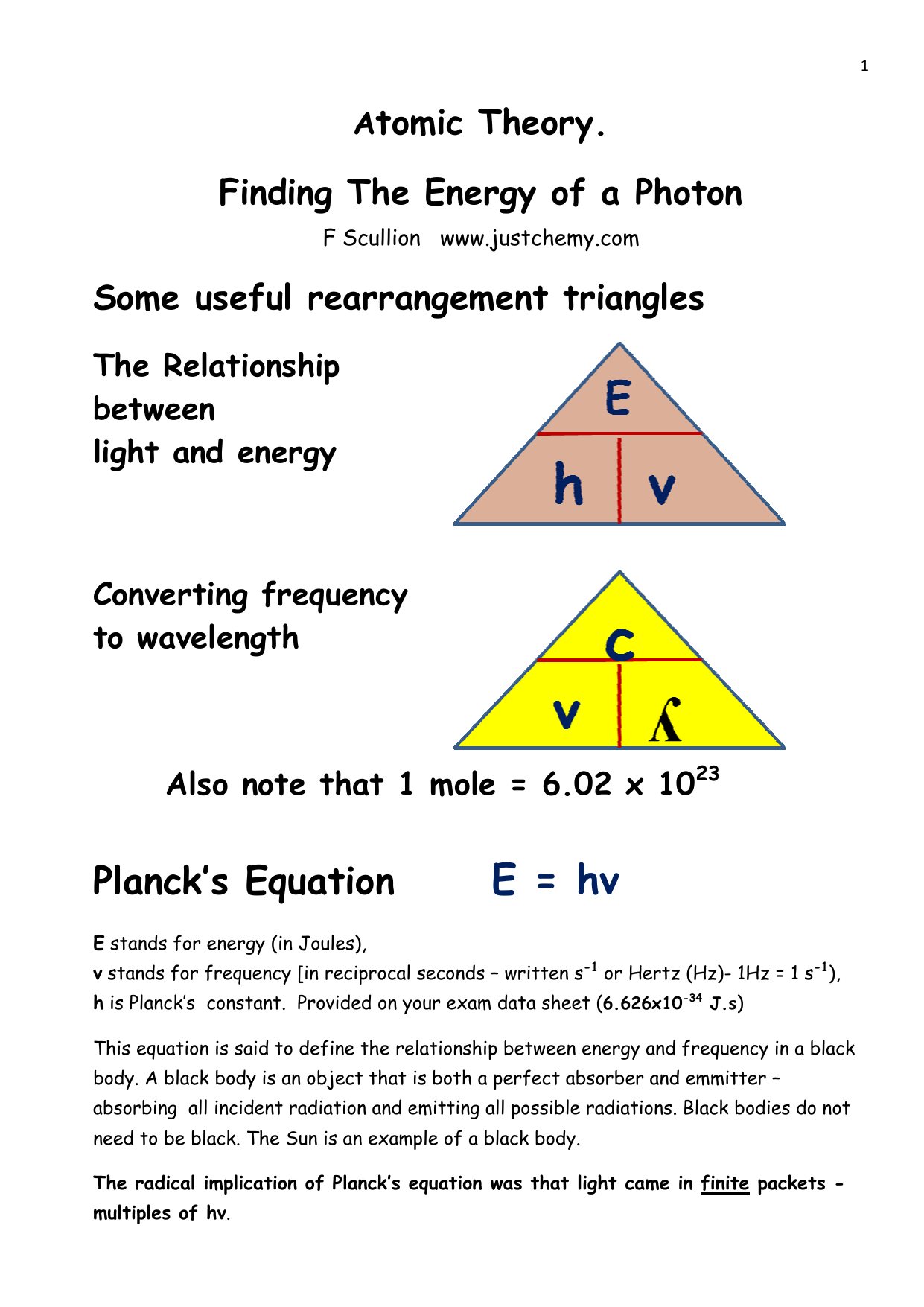
Calculate the energy of one mole of photons of radiation whose frequency is `5 xx 10^(14) Hz`. - YouTube