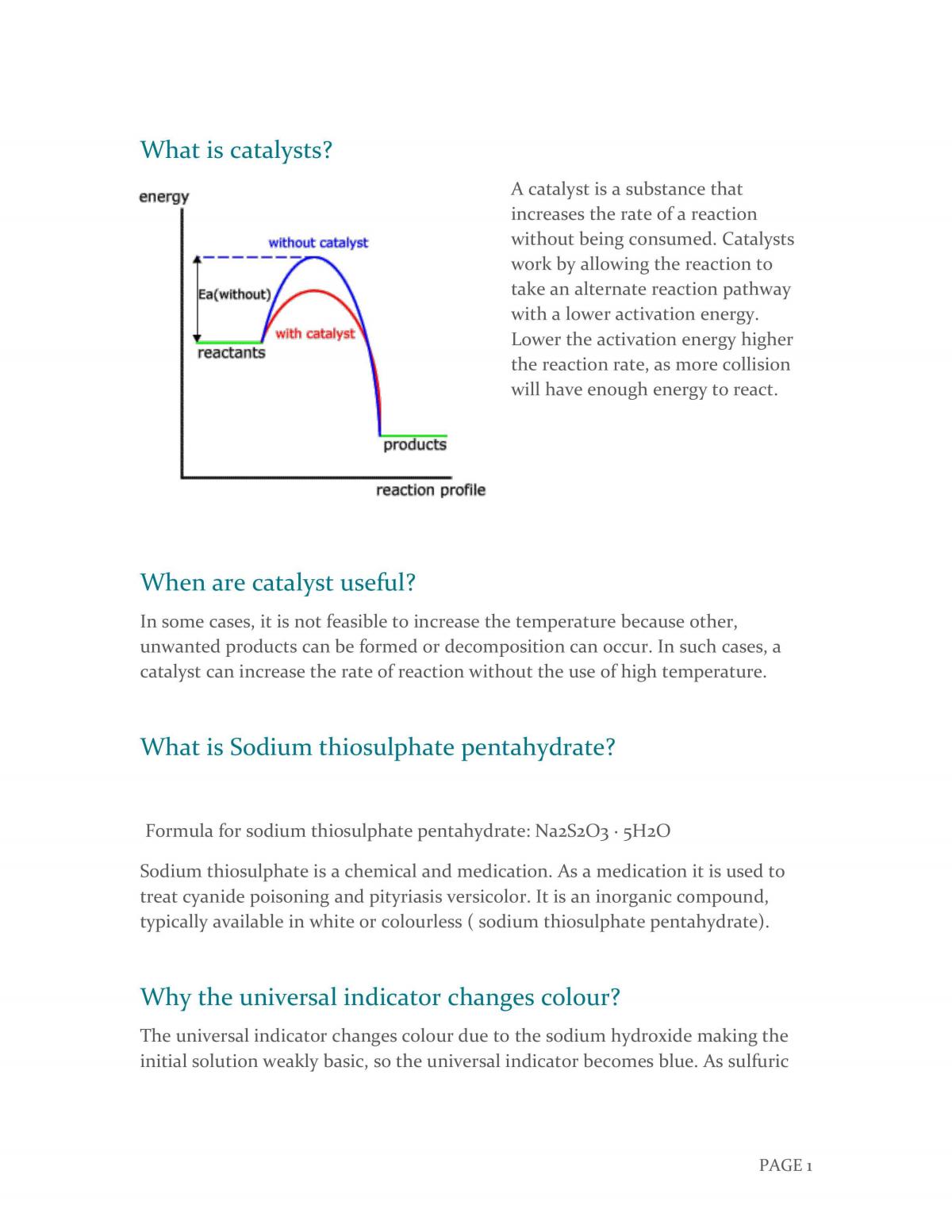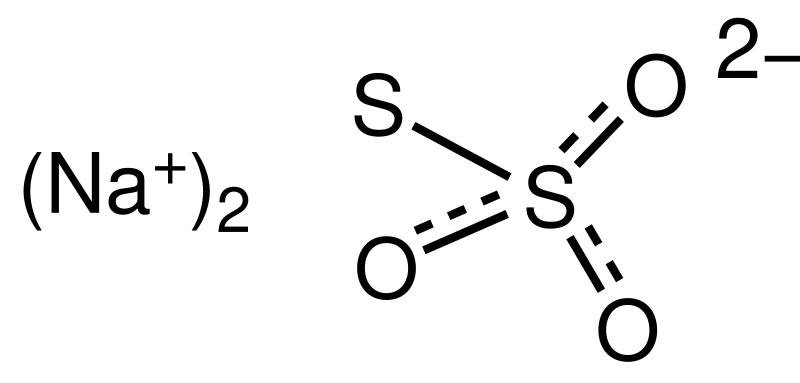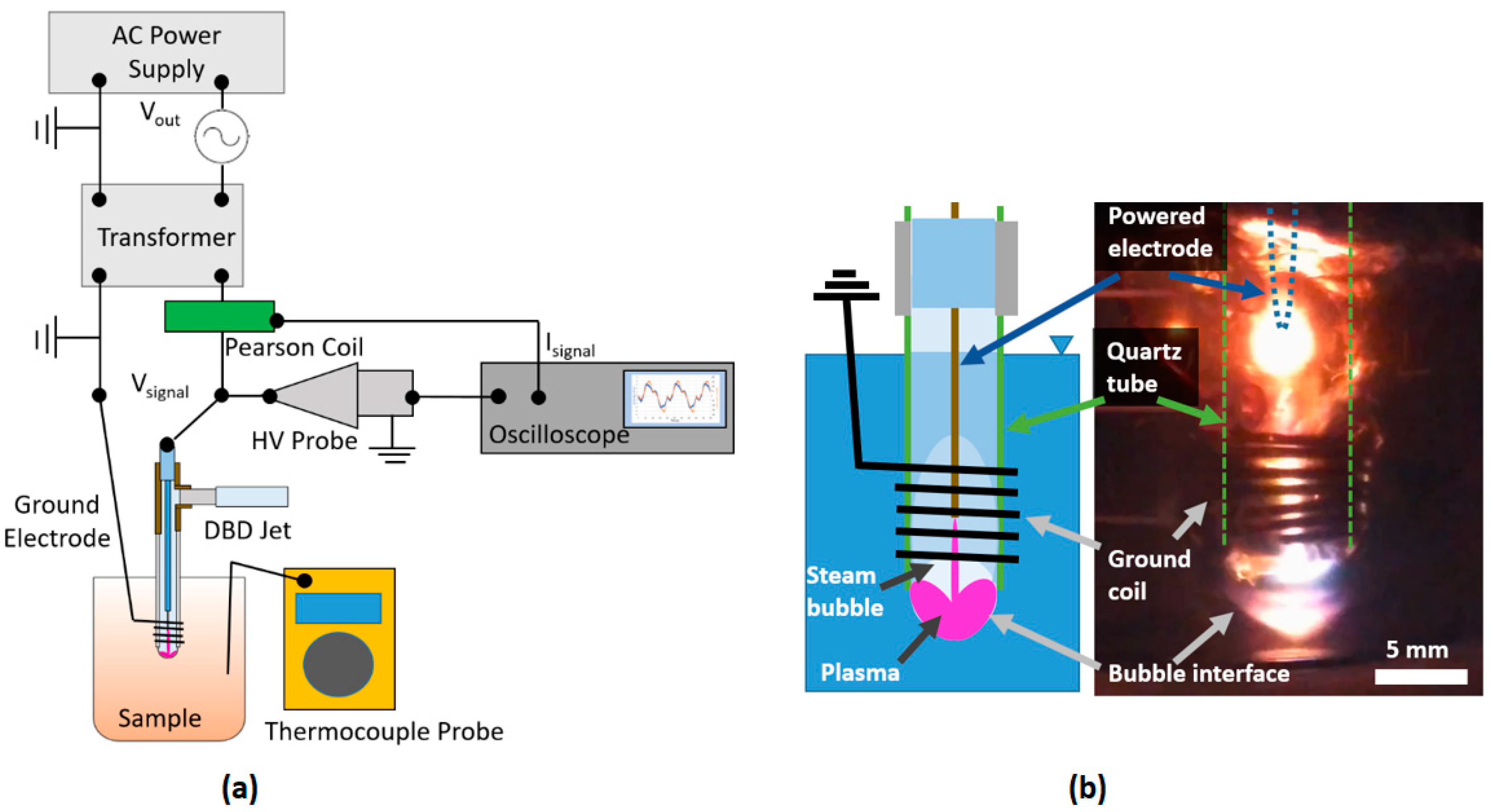
Plasma | Free Full-Text | Hydrogen Peroxide Interference in Chemical Oxygen Demand Assessments of Plasma Treated Waters

Oxygen−Sulfur Species Distribution and Kinetic Analysis in the Hydrogen Peroxide−Thiosulfate System | Inorganic Chemistry

Rapid reaction of sulfide with hydrogen peroxide and formation of different final products by freezing compared to those in solution - Takenaka - 2003 - International Journal of Chemical Kinetics - Wiley Online Library
![PDF] Oxygen-sulfur species distribution and kinetic analysis in the hydrogen peroxide-thiosulfate system. | Semantic Scholar PDF] Oxygen-sulfur species distribution and kinetic analysis in the hydrogen peroxide-thiosulfate system. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ff83a9f4636b352e3a5081f592281c74ca9443e3/4-Figure1-1.png)
PDF] Oxygen-sulfur species distribution and kinetic analysis in the hydrogen peroxide-thiosulfate system. | Semantic Scholar
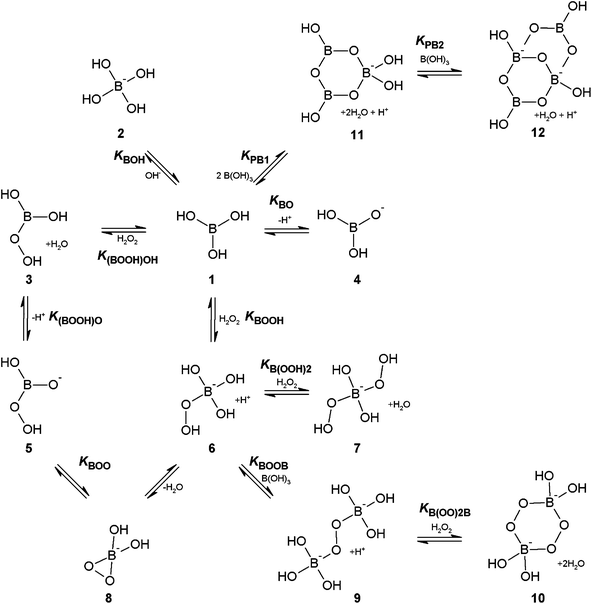
A kinetic and theoretical study of the borate catalysed reactions of hydrogen peroxide : the role of dioxaborirane as the catalytic intermediate for a ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C2OB26842F
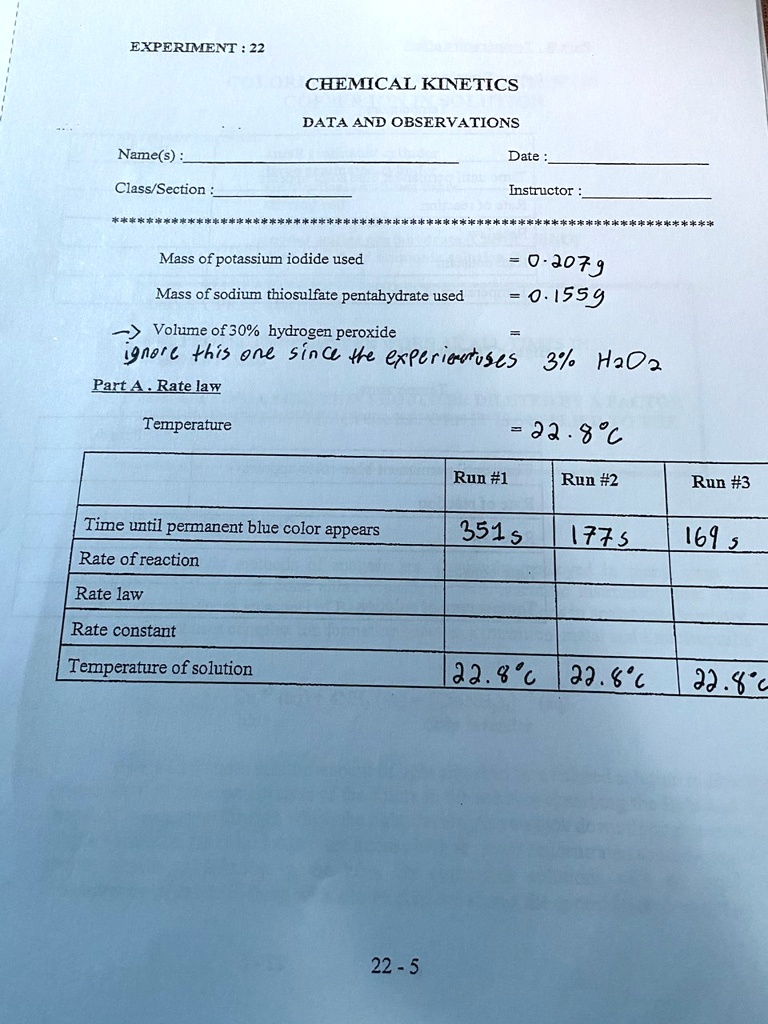
SOLVED: EXPERIMENT CHEMICAL KINETICS DATA AND OBSERVATIONS Name(s) Date Class/Section Instructor #450860a Mass of potassium iodide used 0- J079 0. 1559 Mass of sodium thiosulfate pentahydrate used Volume of 30% hydrogen peroxide
Experiment 5 Kinetics: The Oxidation of Iodide by Hydrogen Peroxide Molecular equation: 2KI(aq) + 2HCl(aq) + H O (aq) I (s) +

Mechanism of the oxidation of thiosulfate with hydrogen peroxide catalyzed by aqua-ethylenediaminetetraacetatoruthenium(III) - ScienceDirect
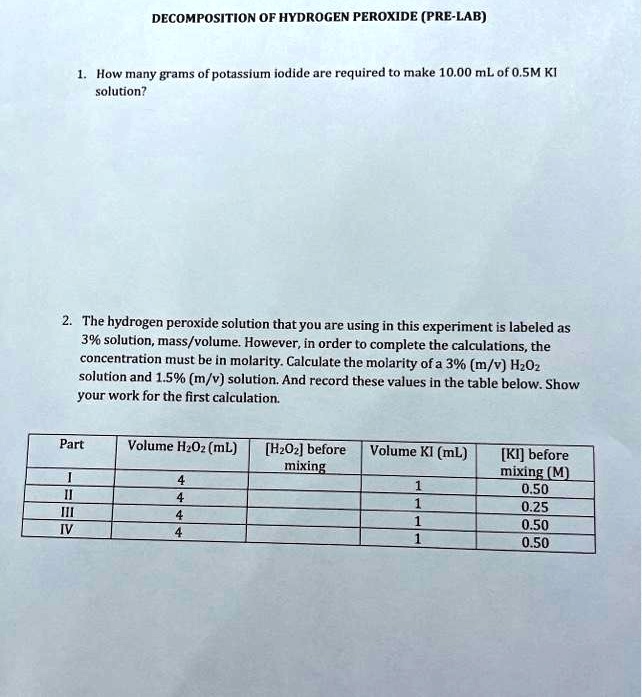
SOLVED: DECOMPOSITION OF HYDROGEN PEROXIDE (PRE-LAB) How many grams of potassium iodide are required to make 10.00 mLofO5M KI solution? The hydrogen peroxide solution thatyou are using in this experiment is labeled

Oxygen−Sulfur Species Distribution and Kinetic Analysis in the Hydrogen Peroxide−Thiosulfate System | Inorganic Chemistry