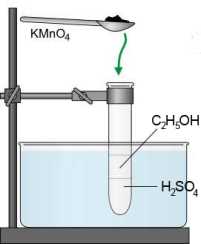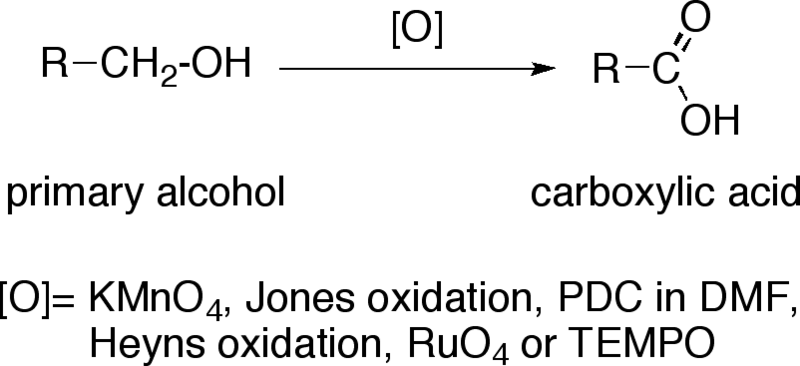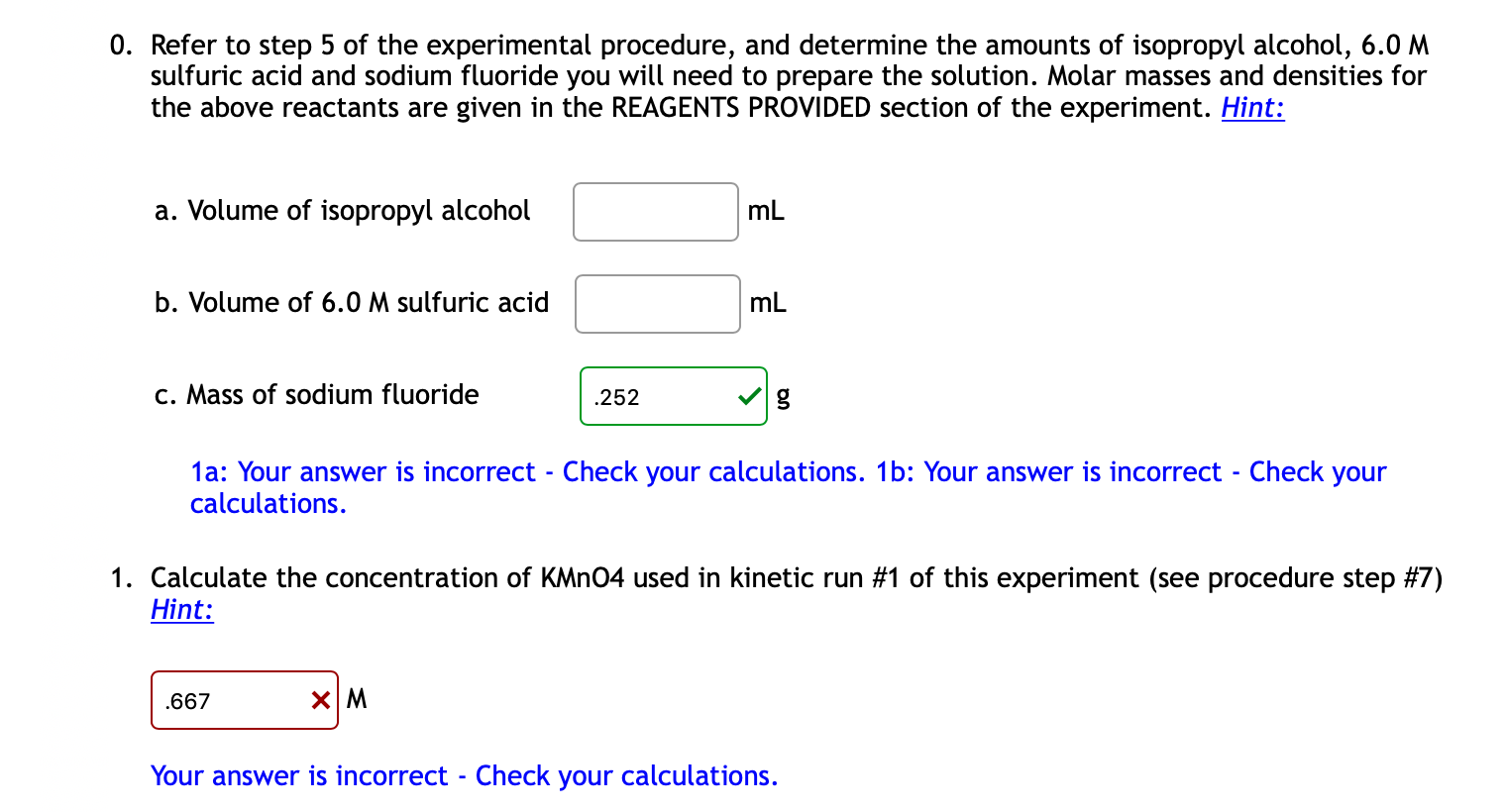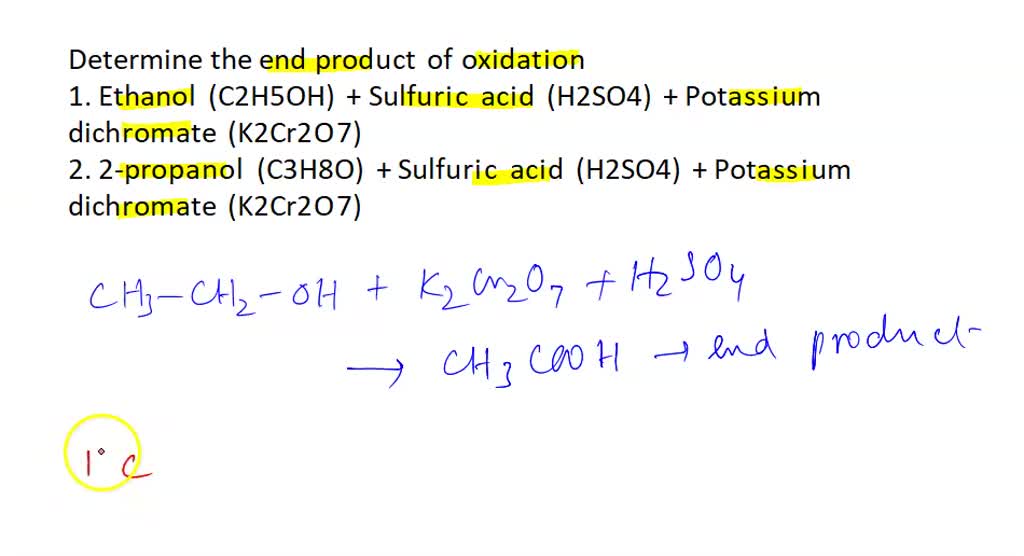
SOLVED: Oxidation of Alcohols Lab Questions Chemicals used Potassium Permanganate,KMnO4(aq) Water, H2O Concentrated Sulfuric Acid, H2SO4(aq) Ethanol Butan-1-ol Butan-2-ol 2-methylpropan-2-ol 1. What is the purpose of adding concentrated Sulfuric Acid in an

Fully Converting Graphite into Graphene Oxide Hydrogels by Preoxidation with Impure Manganese Dioxide | ACS Applied Materials & Interfaces