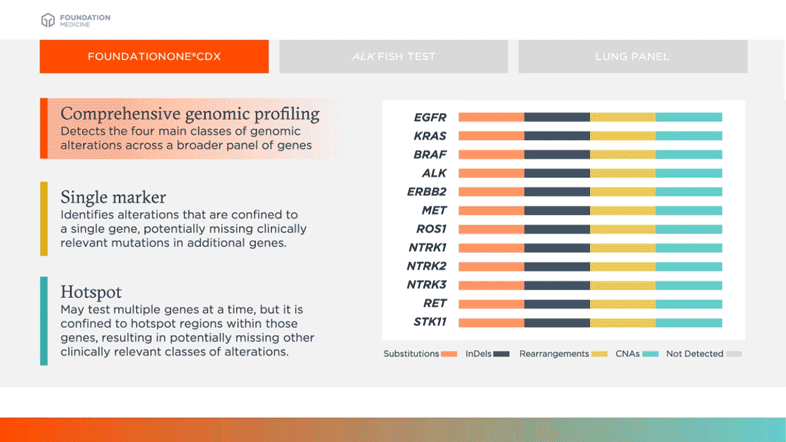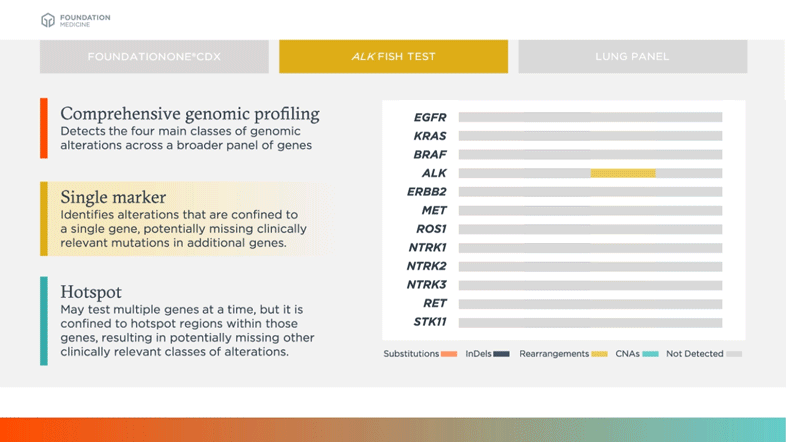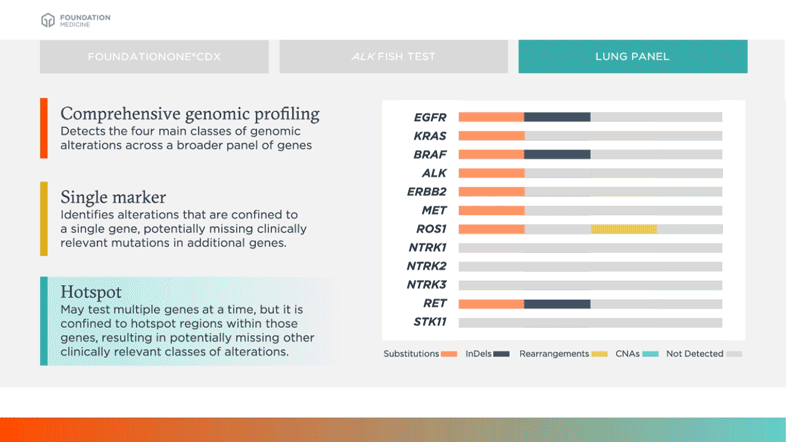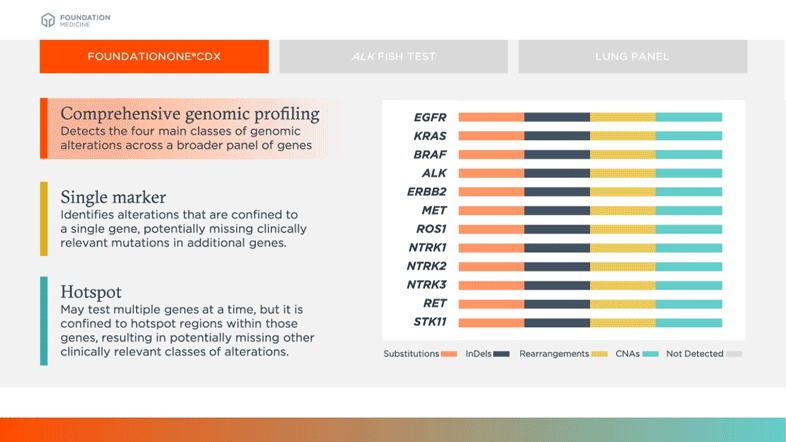
Survey of Precision Oncology Programs Finds Agreement on Testing, Divergence in Care Delivery | Precision Oncology News
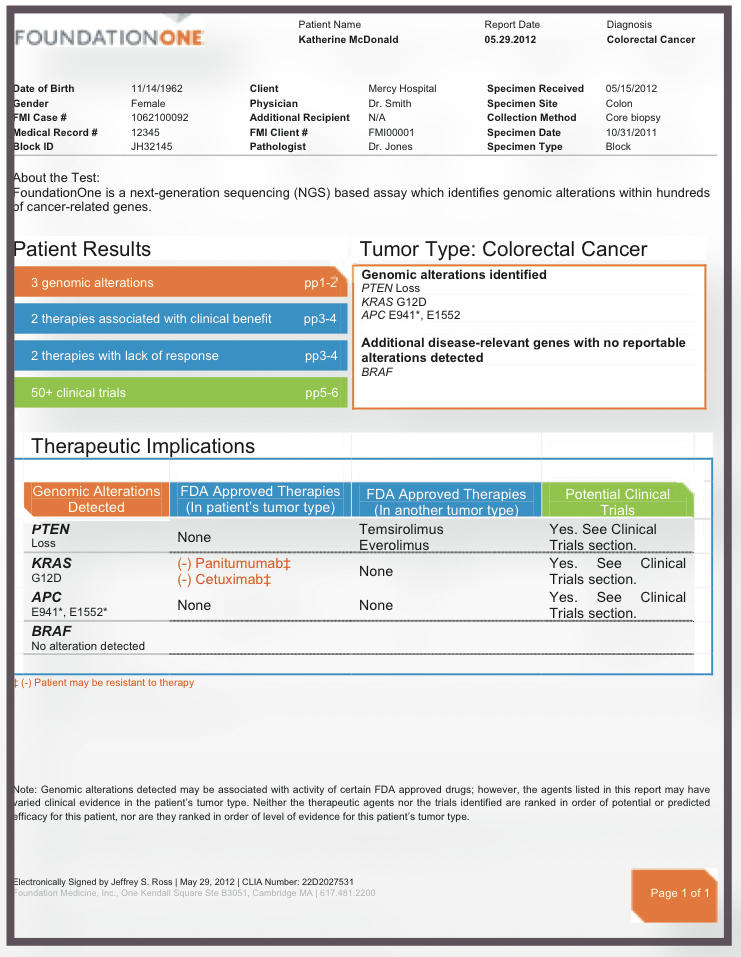
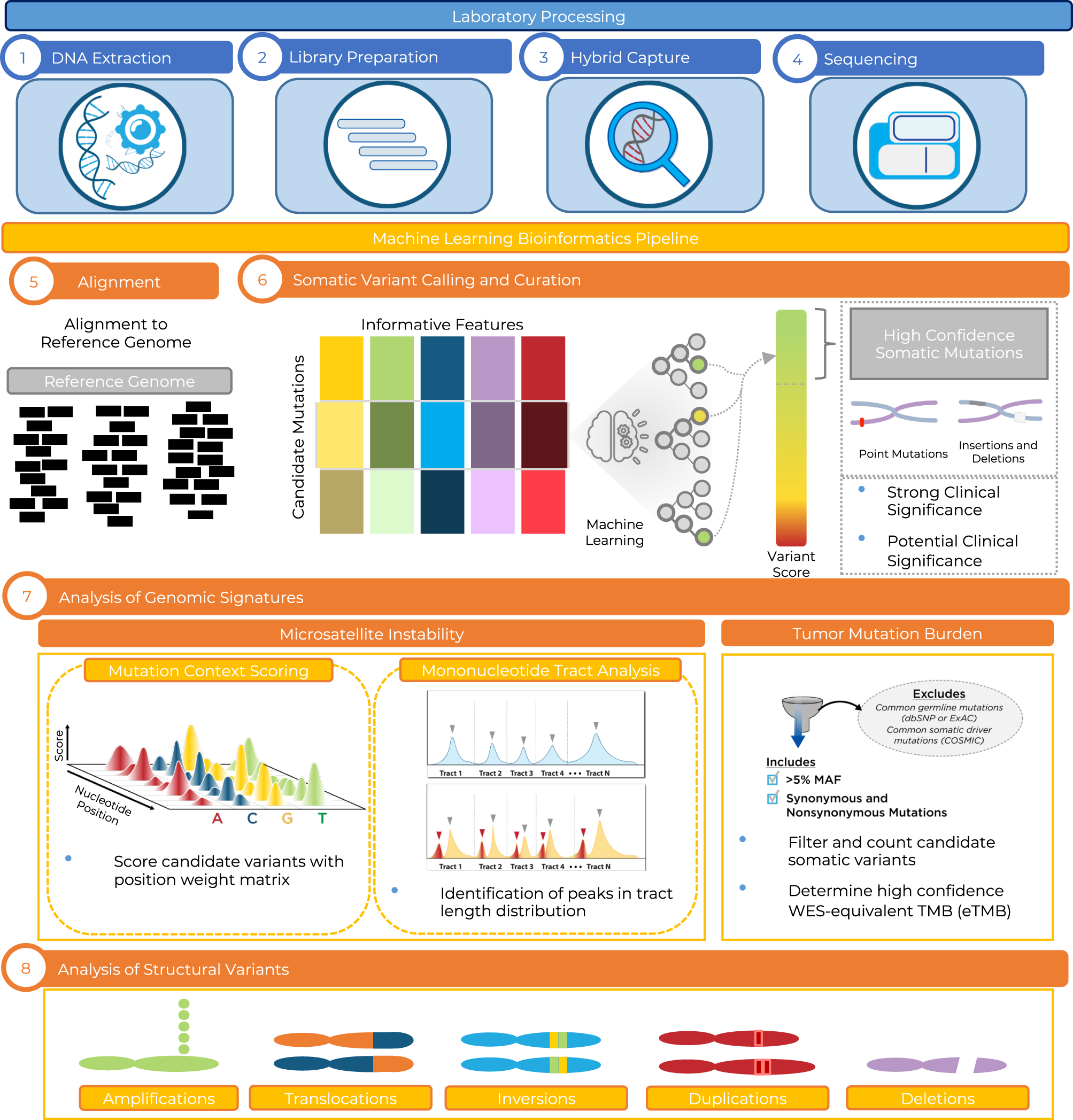
Clinical and analytical validation of FoundationOne®CDx, a comprehensive genomic profiling assay for solid tumors | PLOS ONE

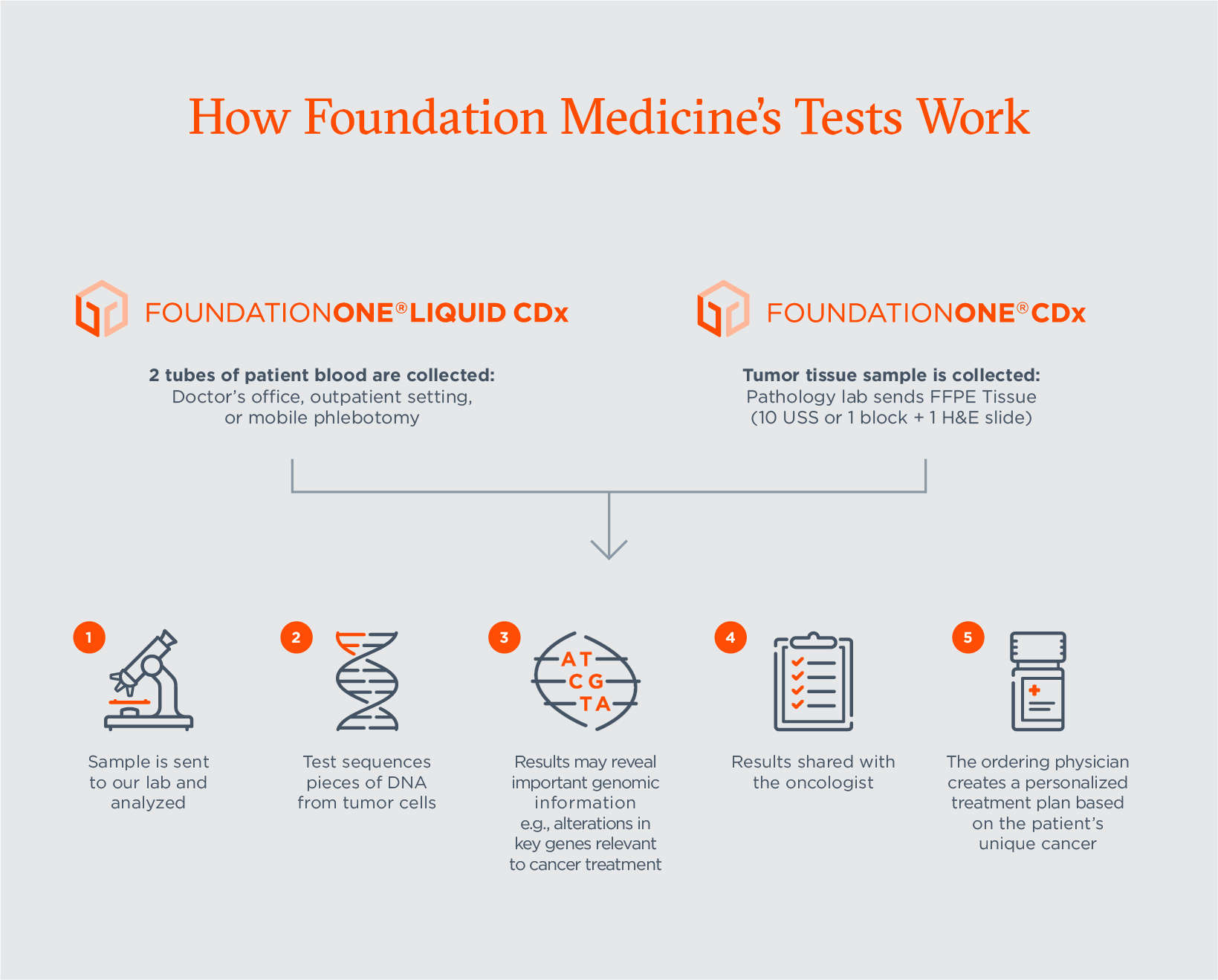
FDA Approves Foundation Medicine's FoundationOne CDx™, the First and Only Comprehensive Genomic Profiling Test for All Solid Tumors Incorporating Multiple Companion Diagnostics | Business Wire

When should we order a next generation sequencing test in a patient with cancer? - eClinicalMedicine

Concordance analysis of microsatellite instability status between polymerase chain reaction based testing and next generation sequencing for solid tumors | Scientific Reports

FDA Approves Foundation Medicine's FoundationOne CDx™, the First and Only Comprehensive Genomic Profiling Test for All Solid Tumors Incorporating Multiple Companion Diagnostics | Business Wire

When should we order a next generation sequencing test in a patient with cancer? - eClinicalMedicine

Analytical Validation of a Hybrid Capture–Based Next-Generation Sequencing Clinical Assay for Genomic Profiling of Cell-Free Circulating Tumor DNA - ScienceDirect
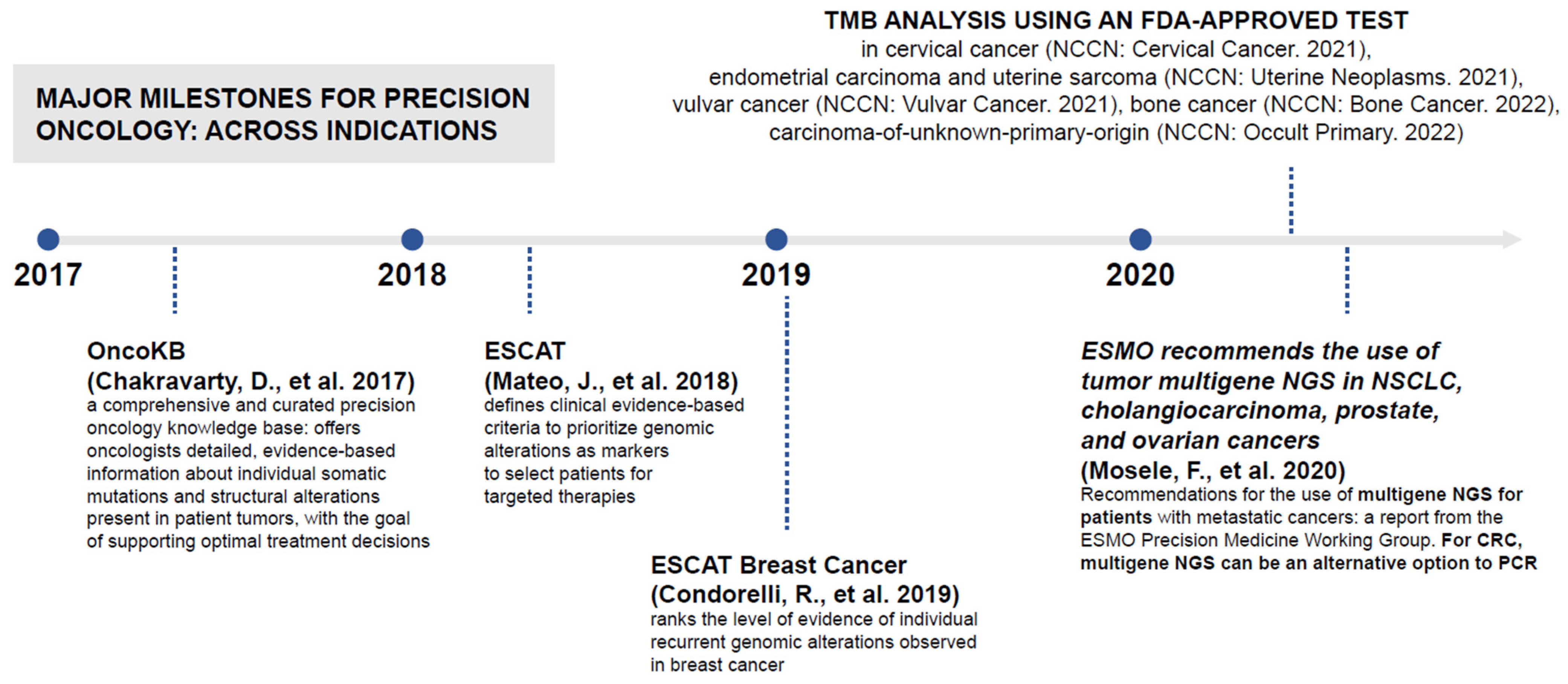
JPM | Free Full-Text | Identifying the Steps Required to Effectively Implement Next-Generation Sequencing in Oncology at a National Level in Europe